Pipeline
Pipeline in Hematologic Malignancies and Autoimmune Diseases: Expanding Indications to Benefit More Patients
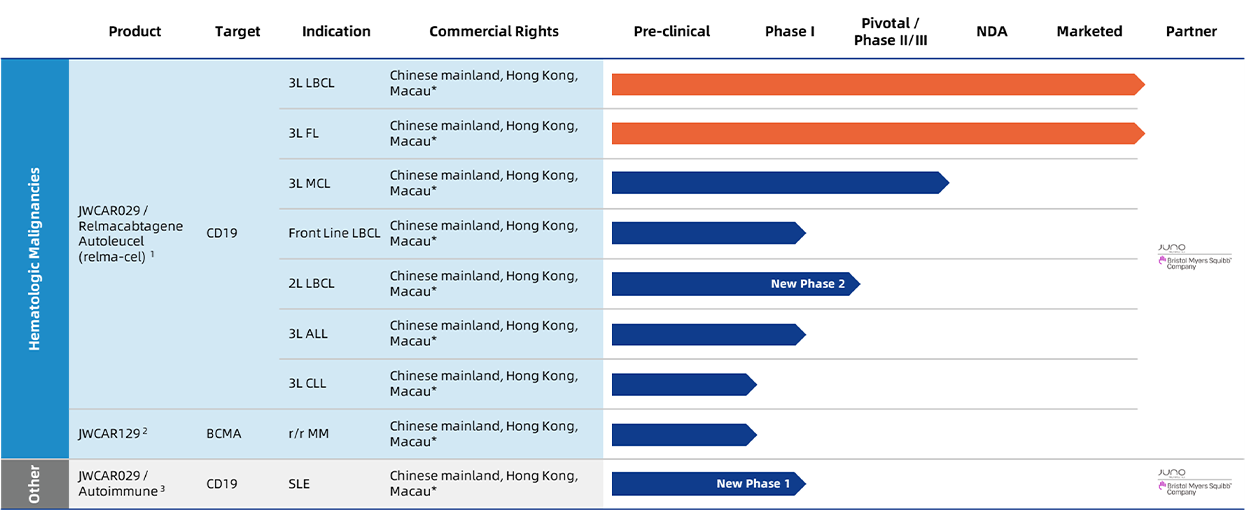
Information as of: March 2023
Abbreviations: LBCL = large B-cell lymphoma; FL = follicular lymphoma; MCL = mantle cell lymphoma; ALL = acute lymphoblastic leukemia; CLL = chronic lymphocytic leukemia; MM = multiple myeloma; NHL = non-Hodgkin lymphoma; SLE = systemic lupus erythematosus.
* Chinese mainland, Hong Kong, Macau and Taiwan refer to Chinese mainland, Hong Kong (China), Macau (China) and Taiwan (China), respectively.
- Relma-cel is based on the same CAR construct as the product lisocabtagene maraleucel (Breyanzi or lisocabtagene or liso-cel) of Juno Therapeutics, which was approved by the U.S. Food and Drug Administration in February 2021.
- JWCAR129 is based on the same CAR construct as Juno Therapeutics’ product orvacabtagene autoleucel (orva-cel).
- SLE is a chronic autoimmune disease characterized by the production of autoantibodies and abnormal B-lymphocyte function. To further extend relma-cel’s potential in broader disease area, we are planning a study to evaluate the safety, tolerability, and pharmacokinetic profile of relma-cel in Chinese patients with moderately or severely active SLE.
Pipeline in Solid Tumors: Targeting High Incidence Diseases in China: HCC, Lung Cancer and More
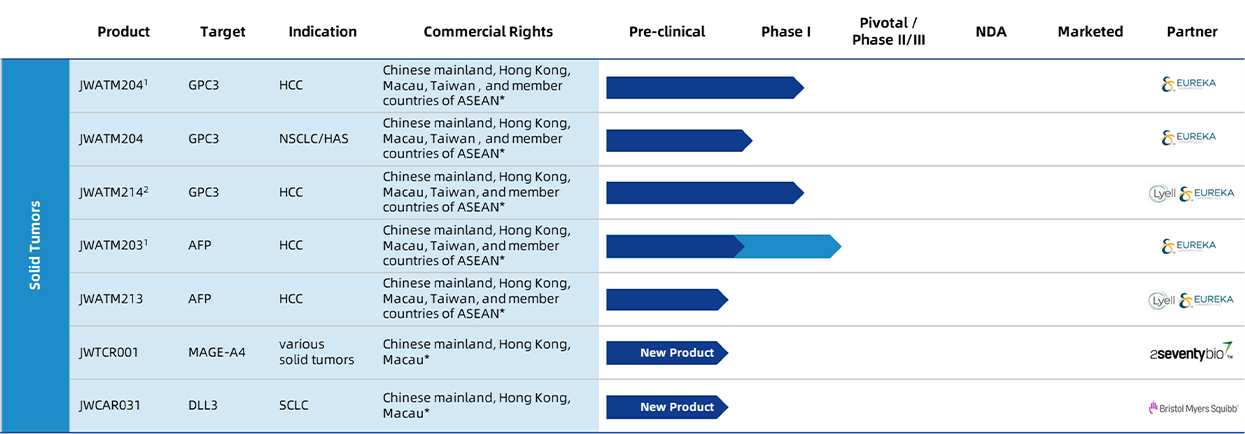
Information as of: March 2023
Abbreviations: HCC = hepatocellular carcinoma; NSCLC = non-small cell lung cancer; AFP = alpha-fetoprotein; GPC3 = glypican-3; r/r = relapsed or refractory; 3L = third-line; 2L = second-line; HAS= hepatoid adenocarcinoma of the stomach;MAGE A4= melanoma associated antigen A4; DLL3= Delta-like ligand 3;
* Chinese mainland, Hong Kong, Macau and Taiwan refer to Chinese mainland, Hong Kong (China), Macau (China) and Taiwan (China), respectively.
- JWATM204 is in a Phase I investigator-initiated trial in China. Eureka’s products based on the CAR constructs underlying JWATM203 and JWATM204 are currently in Phase I/II trials in the US conducted by Eureka under an IND application. In November 2021, the U.S. FDA granted Fast Track Designation to Eureka’s counterpart to JWATM203 for the treatment of hepatoblastoma (HB) and HCC in pediatric patients, as well as “rare pediatric disease designation” for the treatment of HB. In February 2022, the FDA granted Orphan Drug Designation to Eureka’s counterparts to JWATM203 and JWATM 204.
- Developing using Lyell technology.
